E-mail:public_yc@163.com
Study on supramolecular chiral compounds
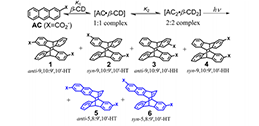
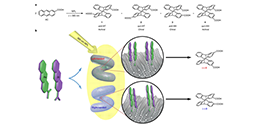
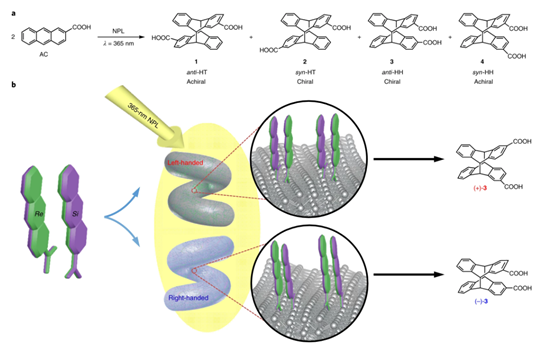
The generation of molecular chirality in the absence of any molecular chiral inductor is challenging. Furthermore, chirality at different hierarchical levels can be transmitted through space and across length scales, mainly from the molecular scale to supramolecular, microscopic, mesoscopic, and macroscopic scales. It is also well established that clockwise or counterclockwise rotation, accompanied by translation along the rotation axis, results in macroscopic shear forces that enable the generation of chiral materials at the macro-, micro- and nanoscales. Here, we report the induction of molecular chirality triggered by macroscopic shear force, mediated by metal nanohelices (NHs): the chiral photochemical (photochirogenic) cyclodimerization of 2-anthracenecarboxylic acid (AC) adsorbed on the surfaces of NHs is enantioselectively controlled by their handedness. It is proved that the macroscopic rotation may be transferred to the molecular level through multi-step transmission, and the chiral bias may be induced.
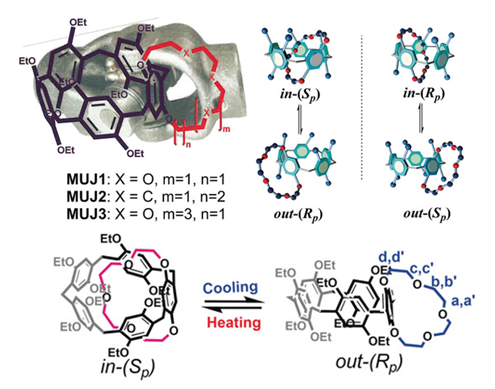
The study of an enantiopure bicyclic pillar[5]arene‐based molecular universal joint (MUJ) by single‐crystal X‐ray diffraction allowed for the first time the unequivocal assignment of the absolute configuration of a planar chiral pillar[5]arene by circular dichroism spectroscopy. Hence is dependent on both the size and nature of the ring and the solvent employed, reflecting the critical balance between the self‐complexation of the ring by pillar[5]arene, the solvation to the excluded ring, and the inclusion of solvent molecules in the cavity. The present results are instructive in particular for a deeper understanding of the temperature effect on natural and artificial chirality‐ and conformation‐related phenomena, for example, the critical dependence of the protein folding/disfolding/binding and the DNA assembling on temperature.
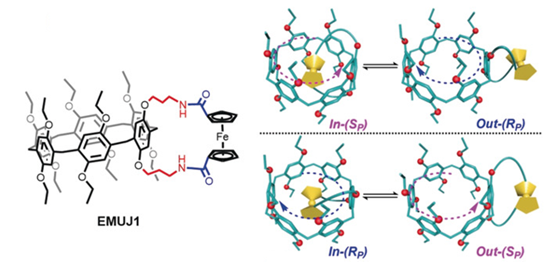
A chiral electrochemically responsive molecular universal joint (EMUJ) was synthesized by fusing a macrocyclic pillar[6]arene (P[6]) to a ferrocene‐based side ring. A single crystal of an enantiopure EMUJ was successfully obtained, which allowed, for the first time, the definitive correlation between the absolute configuration and the circular dichroism spectrum of a P[6] derivative to be determined. The self‐inclusion and self‐exclusion conformational change of the EMUJ led to a chiroptical inversion of the P[6] moiety, which could be manipulated by both solvents and changes in temperature. The EMUJ also displayed a unique redox‐triggered reversible in/out conformational switching, corresponding to an occupation/voidance switching of the P[6] cavity, respectively. This phenomenon is an unprecedented electrochemical manipulation of the capture and release of guest molecules by supramolecular hosts.
Pre:Study on Supramolecular Photochemistry-photodimerization of anthracic acid